User Manual Download.
Arabic
Stakeholder Management Portal
User Manual v1.1
Abbreviations
Establishment: The main establishment that runs any of operations concerning drug supply chain through its administration or any of its stakeholders (manufacturer, supplier, warehouse, hospital or pharmacy)
Stakeholder: Branches or any organization that operates under the establishment�s administration (manufacturer, supplier, warehouse, hospital or pharmacy)
SFDA: The Saudi Food and Drug Authority
DTTS: Drug Track and Trace System
Project summary
The Track and Trace System aims to track the location of each unit of registered human drug that is distributed in Saudi Arabia by defining each location with a Global Location Number (GLN) and each pack of drug with a Global Trade Item Number (GTIN) and a serial number encoded into a GS1 standard DataMatrix.
By this operation, SFDA will be able to increase the patient's safety with combating the counterfeit and falsified drugs by controlling the distribution of recalled and blocked drugs. The system will track each transaction in the supply chain starting from the production or importation to the consumption.
Login to DTTS Stakeholder Management Portal
If you already have an account, enter your username and password then press "Login". If not, click �Register� to register your establishment and stakeholder information.

Register
The establishment must start the registration process by filling its information then details of one of its stakeholders. If the registration request is approved by SFDA, the establishment can proceed with registering its other stakeholders.
The establishment has to upload "Authorization Form" from SFDA that has the delegate�s information along with a copy of hers/his national ID. Please refer to Authorization Form template.
Fill in the establishment information in the first page and one of the stakeholders� information in the next page with the correct information and then click submit to send your request.
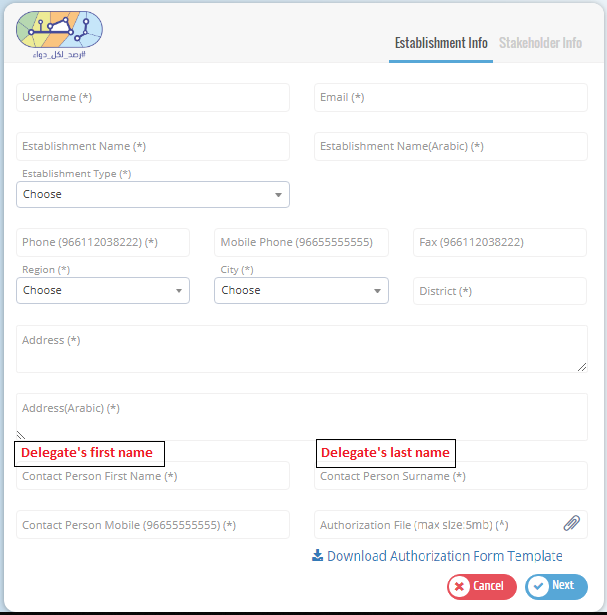
Please use your GLN given from GS1.
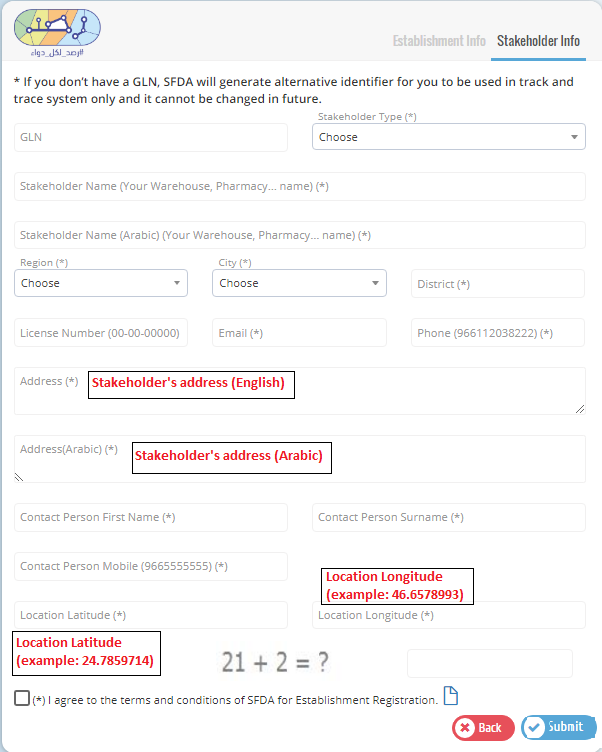
After completing the registration, the establishment user will get an e-mail that contains a link to create a login password.
My Profile
The establishment user can review the establishment profile information by clicking the Establishment's name on the main screen as follows.
Only the "Password" can be changed in My Profile page.

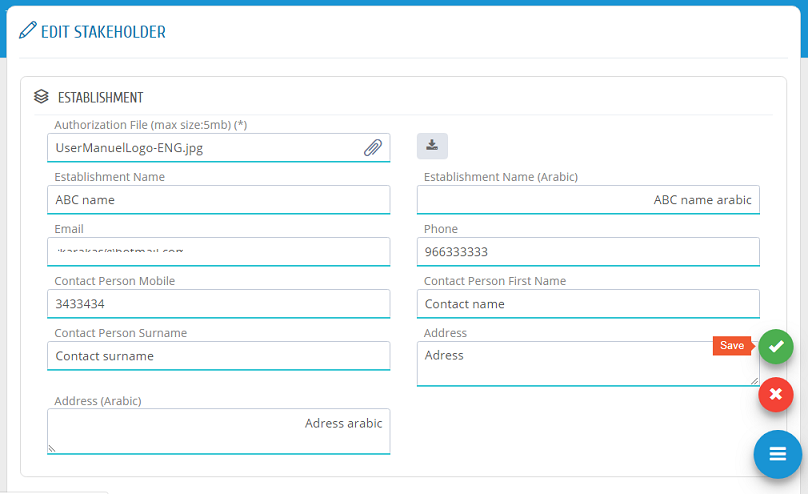
Establishment user can edit the establishment information by clicking �Edit� in Stakeholder list. In Edit Stakeholder Page, the required changes can be edited and saved to submit the change request.
Stakeholder Requests
After creating a password, the responsible person of an establishment can login by e-mail (or username) and password.
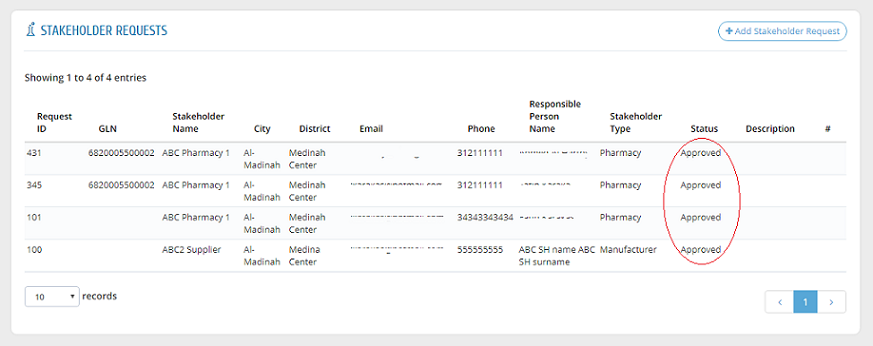
To view the status of a request, please head to "Stakeholder Requests" to find all requests with one of the following statuses:
The "Description" field shows the reason or comment of SFDA about the request if it is rejected or returned for revision.
Add Stakeholder Request
If the establishment registration request is approved, the establishment can register its other stakeholders. Please press "Stakeholder Requests" from the menu then "New Stakeholder" then "Add Stakeholder Request" as shown in the picture.
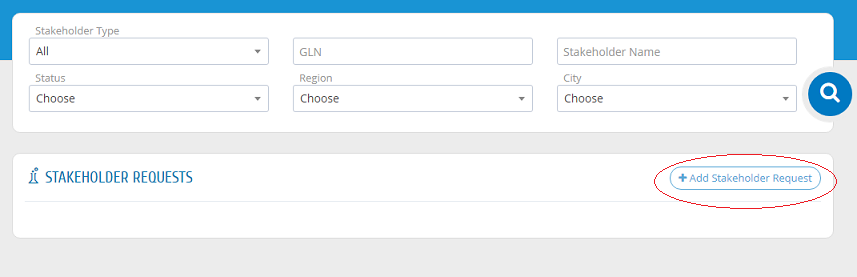
Please use GLN number given from GS1 for each stakeholder.
After filling the required fields, please ensure to �save�.
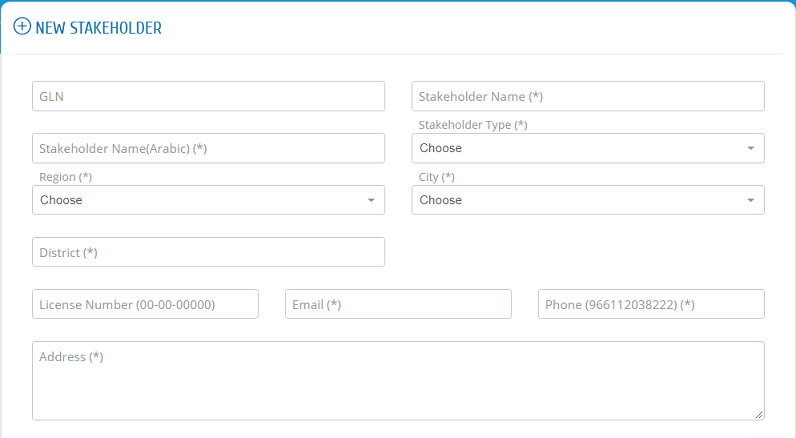
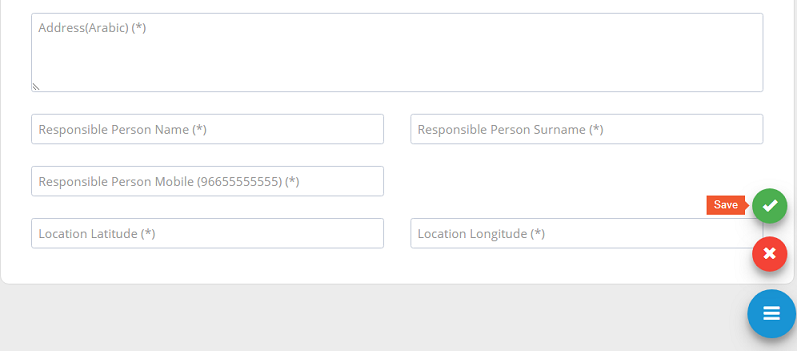
All new stakeholder registration requests or update requests need to be approved by system administration.
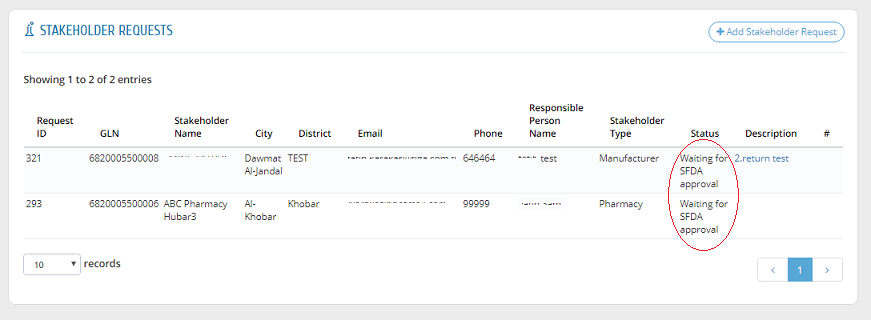
After approving a request by the system administration, a link will be sent to the stakeholder�s email to create username and password. After that, the request is shown as "Approved".
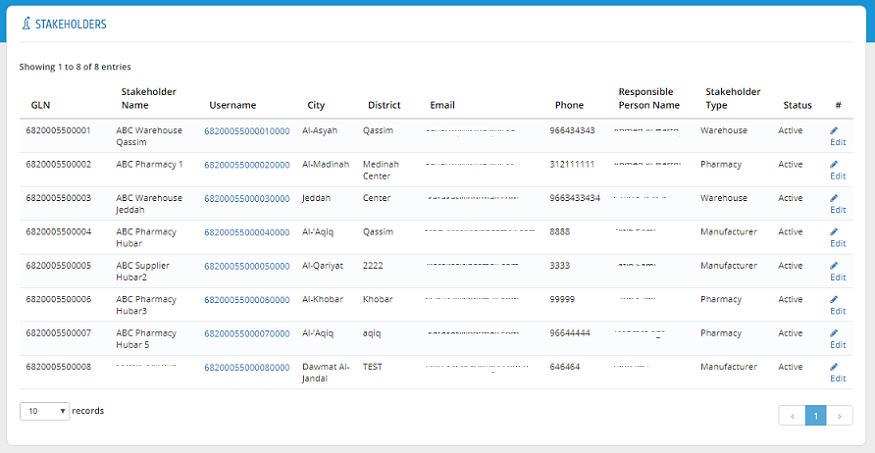
The stakeholder information is now added to the establishment stakeholders list.
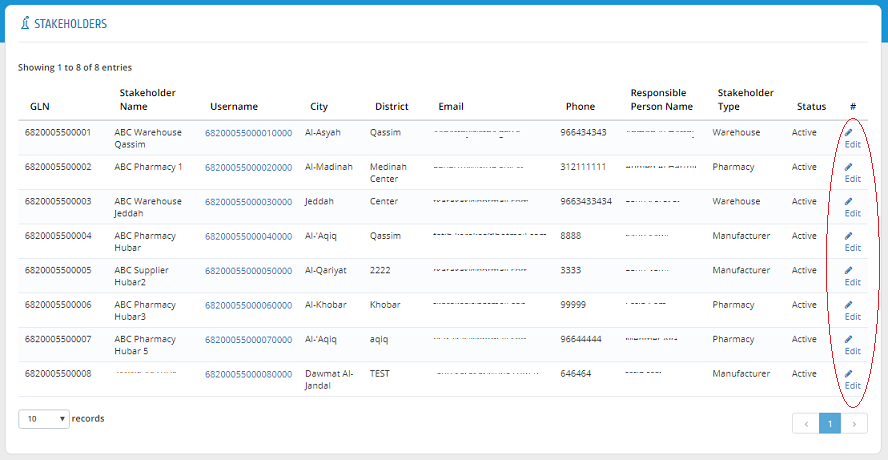
Edit Stakeholder
Stakeholders� information can be edited by clicking �Edit� in Stakeholder list.
In Edit Stakeholder Page, the required changes can be edited and saved to submit the change request.
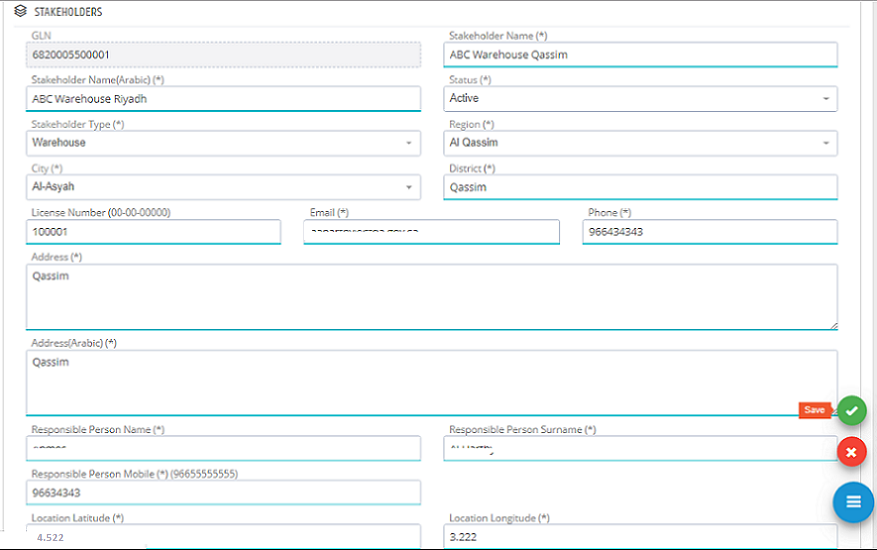
The new request is saved to be approved by the system administration. All new stakeholder register requests or update requests need to be approved by system administration.
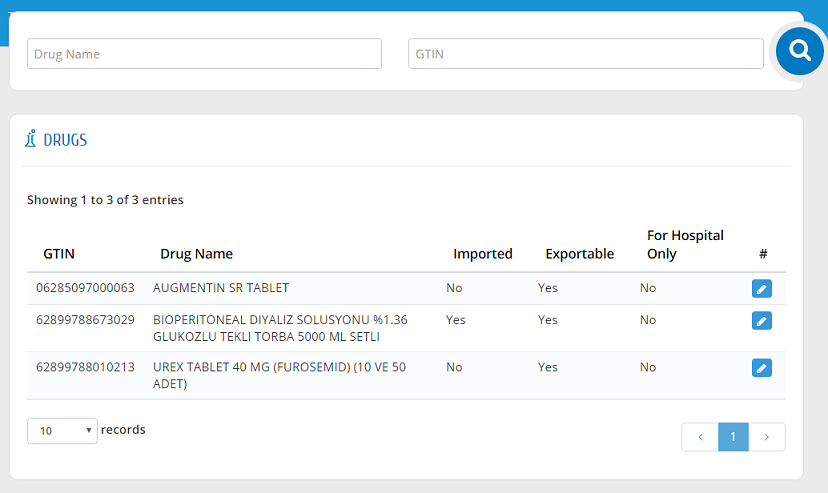
Drugs
Users can view all drugs that are in their establishment�s inventory in this list. Drug List can be accessed by clicking "Drugs" link from the menu. The drugs can be filtered by entering Drug Name or GTIN.
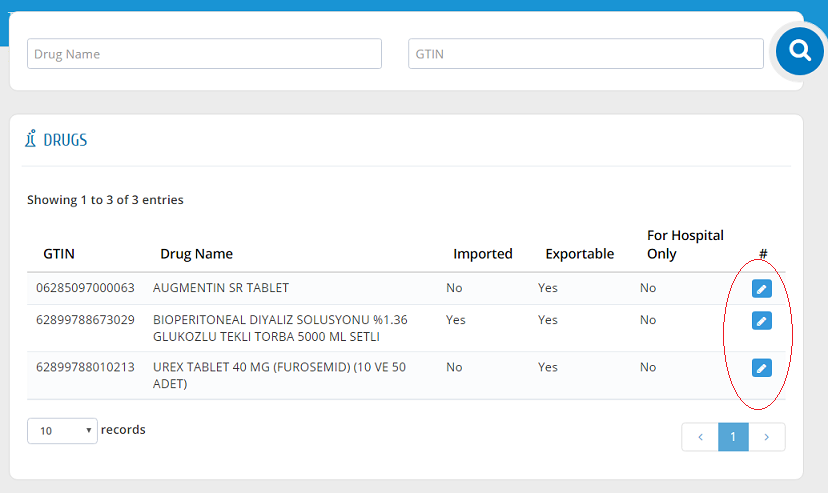
Edit Drugs Information
SFDA will generate random GTIN for drugs that don�t have it. For this reason, suppliers must check their drug information and if necessary, they need to correct GTIN information of these drugs as shown in the picture.
Stakeholders are able to change their drugs' GTIN information with the following conditions:
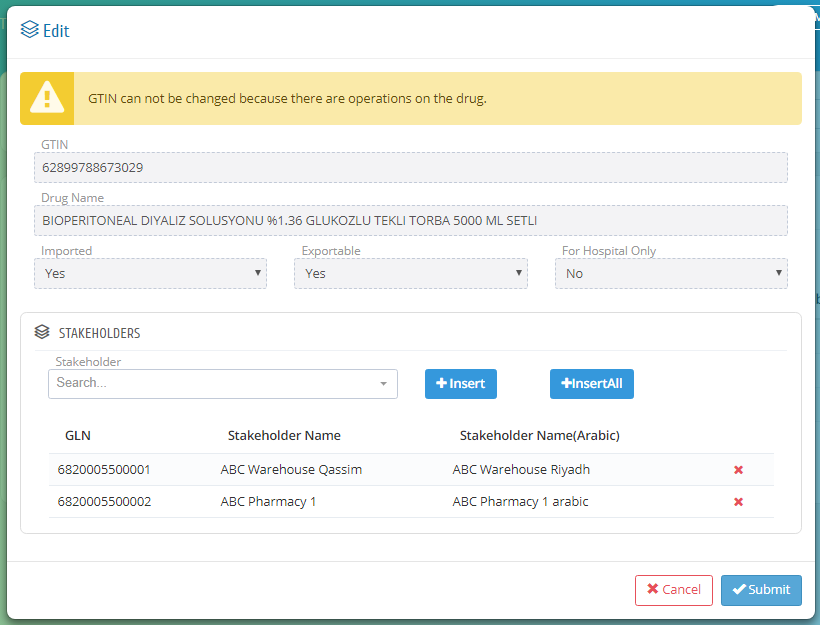
In this page, the establishment can give privilege to its stakeholders to make them supply or import the drugs by searching on the stakeholder and click insert or they can insert all stakeholders under that establishment by clicking insert all.
Reports
Reports can be accessed by clicking "Reports" from the menu. An establishment can find a status or movements of its stakeholders from this option.
Stock Summary
Establishment can see stock status of its stakeholders according to some criteria like Drug Information and Stakeholder type or can select directly the required stakeholder to make a query. Stakeholder Type, GLN, Stakeholder Name, GTIN, Drug Name and Count information are shown in this report.

Stakeholder Operation Summary
Establishment can see operation summary of its stakeholders between selected two dates. Stakeholder Type, GLN, Stakeholder Name, Operation, GTIN, Drug Name and Count information are shown in this report.

Documents
Documents can be accessed by clicking "Documents" from the menu.
Integration Documents
Users can access the integration guide documents by clicking "Integration Guide". Integration documents can also be downloaded by clicking "Download".
